Health Canada Issue. Your firm initiated a recall ie.
 Allergan Textured Breast Implants Recalled What You Need To Know
Allergan Textured Breast Implants Recalled What You Need To Know
JJs stock remained unaffected on Tuesday.

Ideal implant recall 2019. Breast implants arent expected to last a lifetime and breast augmentation may not be. In addition the device involved in the referenced events is a long-term implant. Ideal Saline Post-Approval Studies.
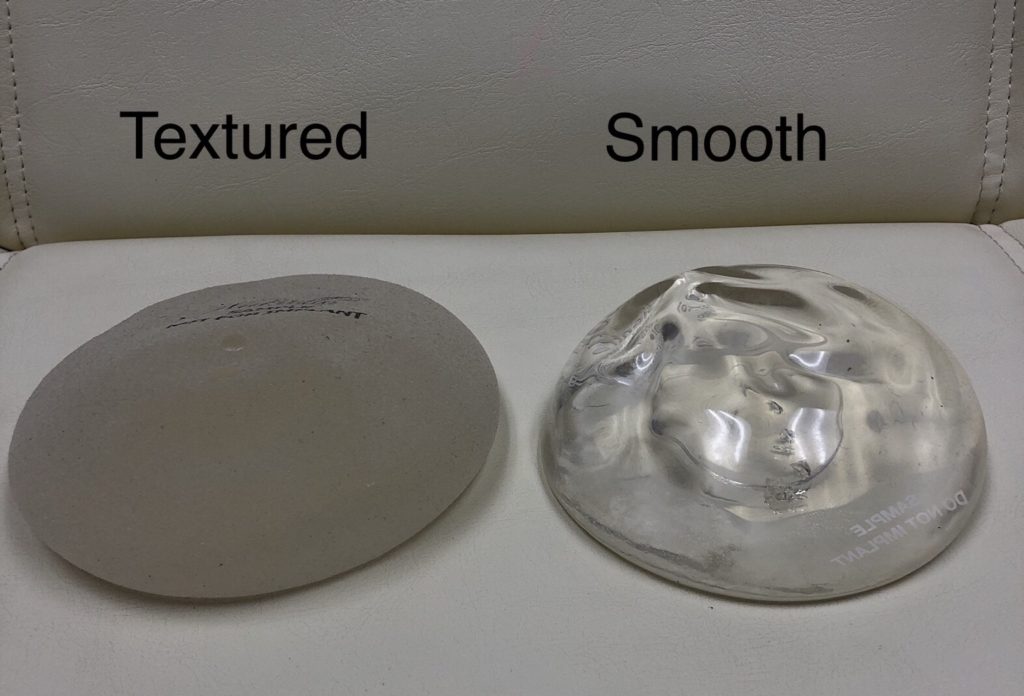
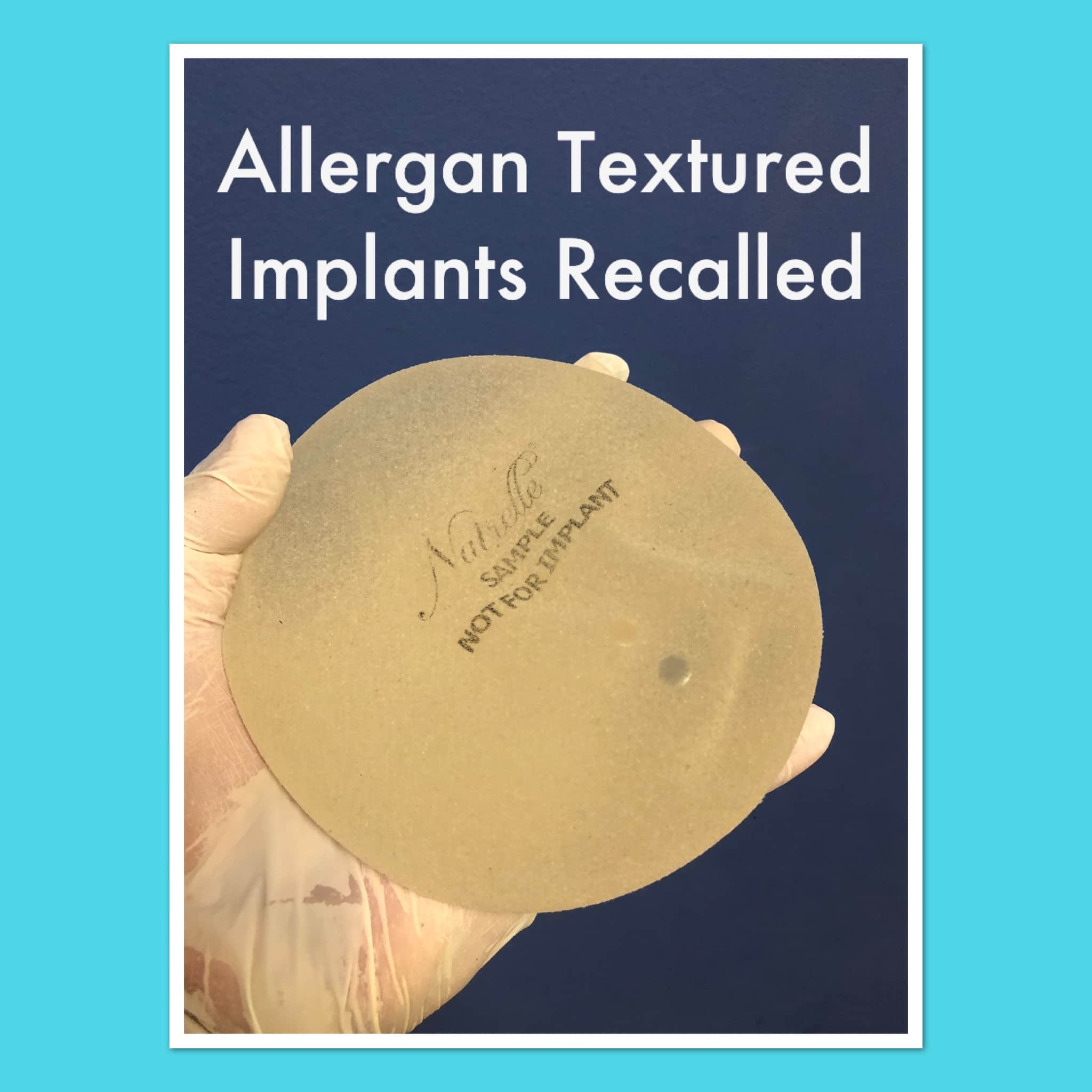
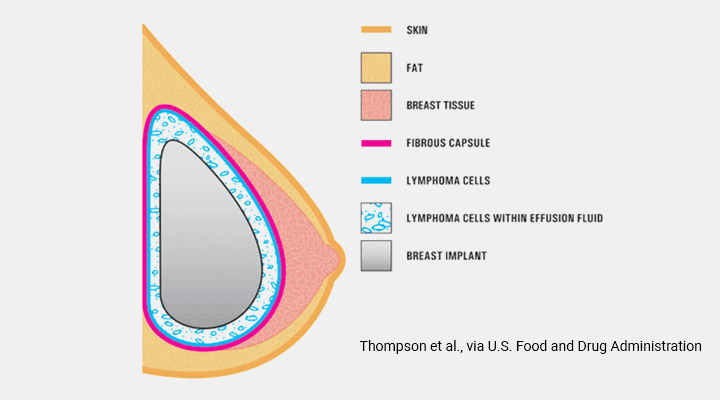
Silicone implants not only have silicone shells but are also filled with silicone gel. 6 7 1012 The explanation for these favorable results is unknown but may be related to the unique technology of the structured implant compared with saline and silicone gel implants. Food and Drug Administration said it called for the removal after new information showed Allergans Biocell breast implants with a textured surface account for a disproportionate share of rare lymphoma cases.
Both types of implants are used to enhance the breasts but they perform very differently both inside and outside the body. When considering breast implants women want a beautiful natural and safe result that doesnt leave them wondering whether their implant has ruptured silently. MASS TORT NEXUS MEDIA A worldwide recall of breast implants by Allergan Inc.
Ideal implant recall over and now on market. But now there is a new generation of implantThe Ideal Implantthats changing the. FDA requested a voluntary recall of its Biocell textured implants in July 2019.
The IDEAL IMPLANT at 6 years had a substantially lower capsular contracture rate and lower failure rate than comparable round saline or silicone gel implants Tables 10 and 11. In the wake of Allergans July 2019 recall of many of its Biocell line of breast implants and tissue expanders two women filed a class-action lawsuit against Allergan in August 2019. In this Monday Nov.
Prosthesis breast inflatable internal saline - Product Code FWM. 23 2015 file photo the Allergan logo appears above a trading post on the floor of the New York Stock Exchange. The IDEAL IMPLANT Structured Breast Implant is the latest implant technology designed by a plastic surgeon to give women the best of both worldsthe beautiful look and natural feel they want with the safety of only saline inside.
What You Need to Know Written ByStacy Simon July 25 2019 The pharmaceutical company Allergan has recalled all BioCell textured breast implants at the request of the US Food and Drug Administration FDA because they have been linked to a rare type of lymphoma. Ideal Implants Returning to Market. Medical Device Recall Subcategory.
ASPS President Alan Matarasso thinks the entire textured implant market will likely be painted with the same brush as the macrotextured products being recalled by Allergan he told MedTech Dive in an interview. Issued a worldwide recall Wednesday for certain textured models after regulators alerted the company to a heightened cancer risk with the devices. Hamas explains the IDEAL IMPLANT Structured Breast Implant contains a novel internal structure that gives it the natural feel and look of silicone gel implants but with only saline inside for a womans peace of mind Inside the IDEAL IMPLANT Structured Breast Implant there are two chambers with baffled layers to control the flow of saline.
March 13 2019 Posting date. Breast Implant Recall. Conduct a Focus Group Study to evaluate whether Ideal Saline-filled breast implants for Breast Augmentation Surgery brochure effectively communicate the risks and benefits of breast implant surgery to women interested in primary or revision breast augmentation according to the protocol version dated 31814.
According to the FDA the other two manufacturers whose breast implants are approved in the United States are Allergan. By MATTHEW PERRONE July 24 2019 WASHINGTON AP Breast implant maker Allergan Inc. May 10 2019 Type of communication.
Was issued Wednesday for textured models because of a link to a rare form of cancer. On Wednesday July 24 2019 the medical device maker. Medical Device Hazard classification.
Although Allergan has committed to paying for the replacement of its recalled devices it hasnt offered to cover the surgery. Ideal implants arent linked to BIA-ALCL a rare type of cancer associated with textured implants. Type II Source of recall.
Ideal Implant Structured Breast. But in the US only about 10 of breast implants. As the New York Times reports the FDA asked Allergan to voluntarily recall all Biocell textured implants.
Breast implant associated anaplastic large cell lymphoma BIA-ALCL. The Ideal implant has not been studied for use in breast reconstruction and therefore isnt indicated for primary breast reconstruction revision breast reconstruction or if there will be radiation of the breast. Z-2219-2019 for the referenced deflation malfunctions.
Ideal Implant Structured Breast Implant 2019-03-13 Report a Concern. September 15 2019. An FDA recall on Allergans Biocell textured implants may affect some cross-fit trainers and yoga instructors in Los Angeles but only about five percent of breast implants sold in the US.
The Ideal implant was temporarily unavailable in order to get further approval by the FDA after the company voluntarily identified a small issue with the valve. Ideal Implant Structured Breast Implant.
 Breast Augmentation Nyc Best Breast Implants New York City
Breast Augmentation Nyc Best Breast Implants New York City
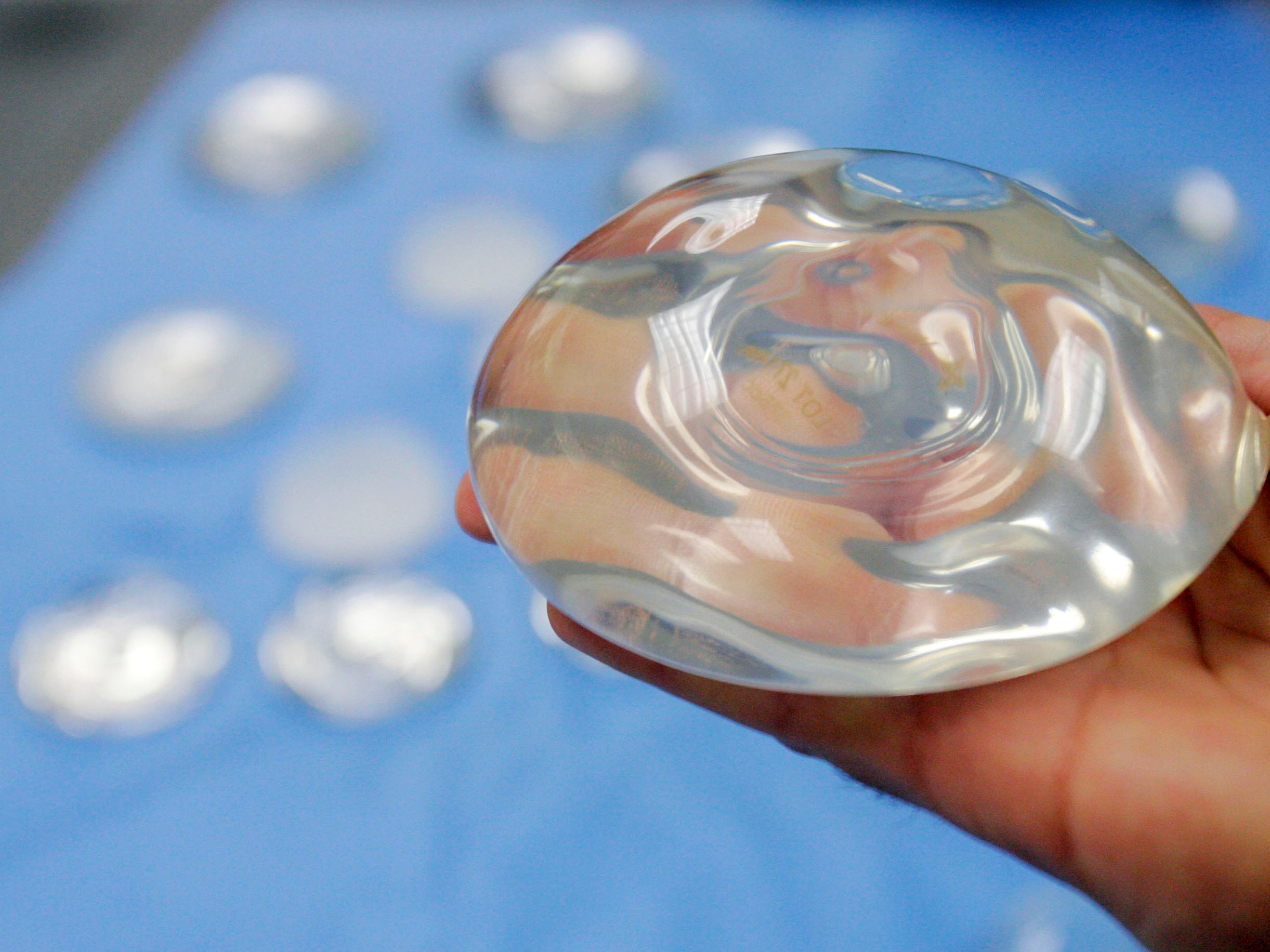 Photos Show The Astonishing Reality Of What Breast Implants Look Like After They Ve Been Removed From The Body
Photos Show The Astonishing Reality Of What Breast Implants Look Like After They Ve Been Removed From The Body
 Allergan Textured Breast Implants Recalled What You Need To Know
Allergan Textured Breast Implants Recalled What You Need To Know
 Allergan Textured Breast Implants Recalled What You Need To Know
Allergan Textured Breast Implants Recalled What You Need To Know
 Allergan Textured Breast Implants Recalled What You Need To Know
Allergan Textured Breast Implants Recalled What You Need To Know
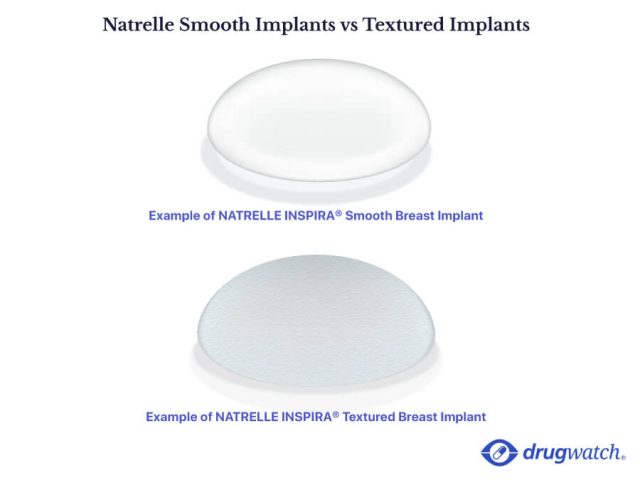 Allergan Breast Implant Recalls List Of Affected Models
Allergan Breast Implant Recalls List Of Affected Models
 Is An Ideal Implant Really An Ideal Implant
Is An Ideal Implant Really An Ideal Implant
 Your Guide To Breast Implant Safety Concerns And Recalls 2019 Allure
Your Guide To Breast Implant Safety Concerns And Recalls 2019 Allure
 Ideal Implant In The News Ideal Implant
Ideal Implant In The News Ideal Implant
 Breast Implant Recalled After Link To More Rare Cancer Cases
Breast Implant Recalled After Link To More Rare Cancer Cases
 How The Risky Business Of Breast Implants Affects Millions Of Women And Big Pharma Fortune
How The Risky Business Of Breast Implants Affects Millions Of Women And Big Pharma Fortune
 Breast Implants Being Recalled What To Know
Breast Implants Being Recalled What To Know
 How The Risky Business Of Breast Implants Affects Millions Of Women And Big Pharma Fortune
How The Risky Business Of Breast Implants Affects Millions Of Women And Big Pharma Fortune
Comments
Post a Comment