Join NPINs new social community to connect share and collaborate. Florida which still leads the nation in new cases has seen those case counts fall 12 from the previous week.
 Where Are We On A Coronavirus Vaccine And What S Next Video
Where Are We On A Coronavirus Vaccine And What S Next Video
The spectrum of medical therapies to treat coronavirus disease 2019 COVID-19 is growing and evolving rapidly including both drugs approved by US.
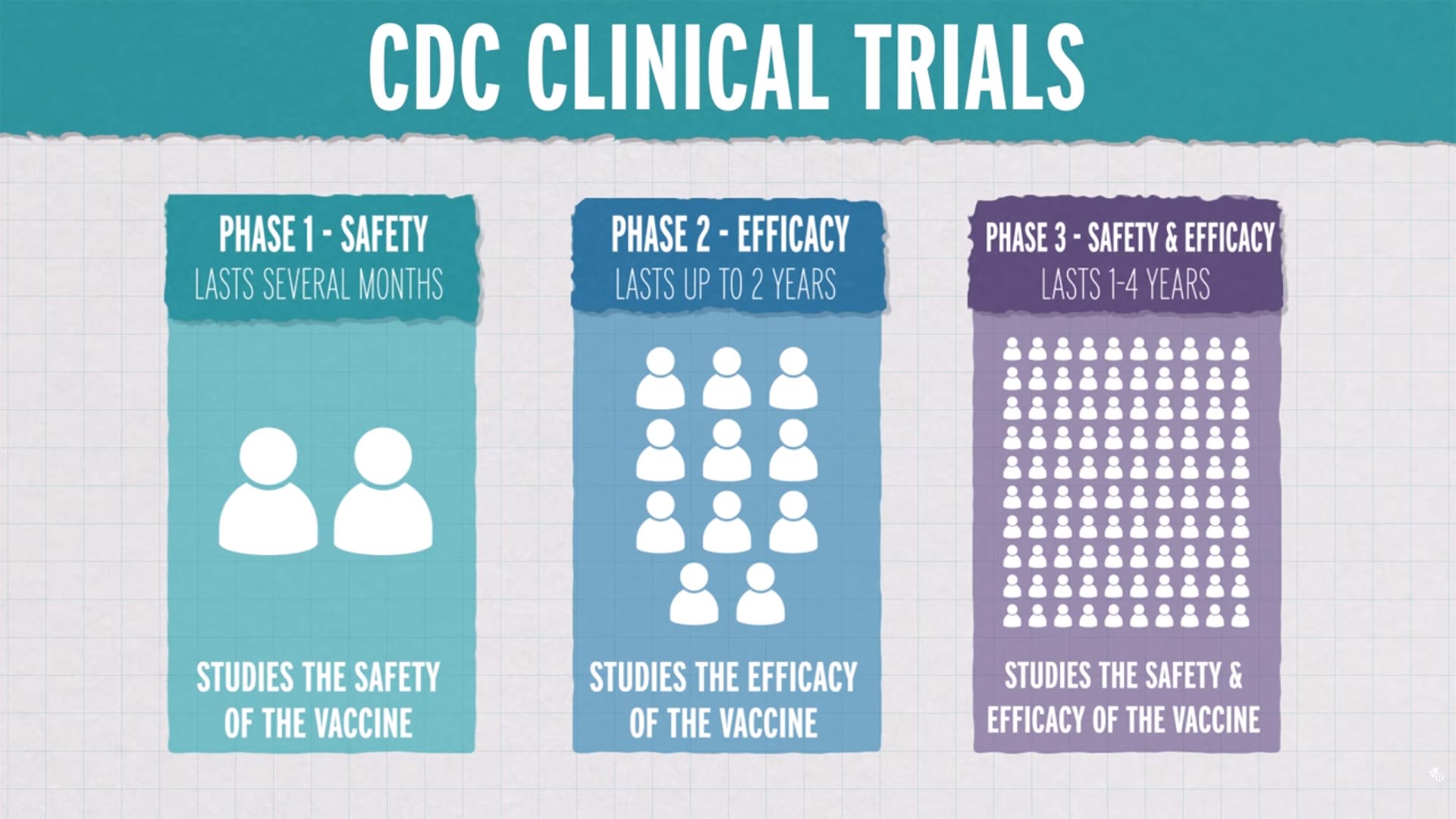
Cdc clinical trials. ClinicalTrialsgov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Search Tips and Examples. Introducing NPINs Social Community.
CDC will continue to provide updates as we learn more about the safety of the JJJanssen vaccine in real-world conditions. A review of safety monitoring data found that 97 of reported reactions after vaccine receipt were nonserious consistent with preauthorization clinical trials data The CDC counted 17. In clinical trials side effects were common within 7 days of getting vaccinated but were mostly mild to moderate.
Review of safety monitoring data found that 97 of reported reactions after vaccine receipt were nonserious consistent with preauthorization clinical trials data. They help doctors learn about cancer and develop better treatments that can help you and other people in the future. Explore 375905 research studies in all 50 states and in 220 countries.
Clinical trials are very important. The CDC said it was the first real-world findings in the United States confirming the clinical trial data that showed the vaccines prevented severe COVID-19. Nearly 8 million doses of the Janssen COVID-19 vaccine had been administered.
A COVID 19 after administration of the study preparation to determine the effectiveness of chlorine. Food and Drug Administration FDA and drugs made available under FDA emergency use authorization EUA. You can enter a word or a phrase such as the name of a medical condition or an intervention.
A clinical trial is a scientific study to find out which treatments or devices are safe and if they work well. NIH COVID-19 Treatment Guidelines Websiteexternal icon NIH Therapeutic Management of Adults With COVID-19external icon. CDC strongly encourages clinicians patients and their advocates and health system administrators to regularly consult the.
This randomized controlled clinical trial will compare standard of care treatment to DPS in. All Phase 3 clinical trials of candidate vaccines supported through OWS are overseen by a common DSMB developed in consultation with the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines ACTIV initiative. Talk sexual health services with other STD prevention professionals.
A total of 17 thrombotic events with thrombocytopenia were detected including three non-CVST events. Use AND in uppercase to search for multiple terms. Their treatment guidelines are updated regularly based on new data.
For more information see How to Search. A new CDC study provides strong evidence that mRNA COVID-19 vaccines are highly effective in preventing SARS-CoV-2 infections in real-world conditions among health care personnel first responders and other essential workers. Click on the links below to practice some sample searches.
Side effects were more common in people 1859 years old compared to people 60 years and older. Listing of current research opportunities at the Centers for Disease Control and Prevention CDC that offer hands-on laboratory experiences and internships for university students and faculty in support of CDCs mission to protect to protect America from health. Clinical trials are underway for vaccinating children as young as 6 months old.
The National Institutes of Health NIH provides treatment and management recommendations for COVID-19 patients. The research will be carried out between April and June 2020 with a quasi-experimental design in two health care centers on a sample of twenty 20 patients through direct intervention who will measure the changes in the manifest symptoms of infection and negativity.
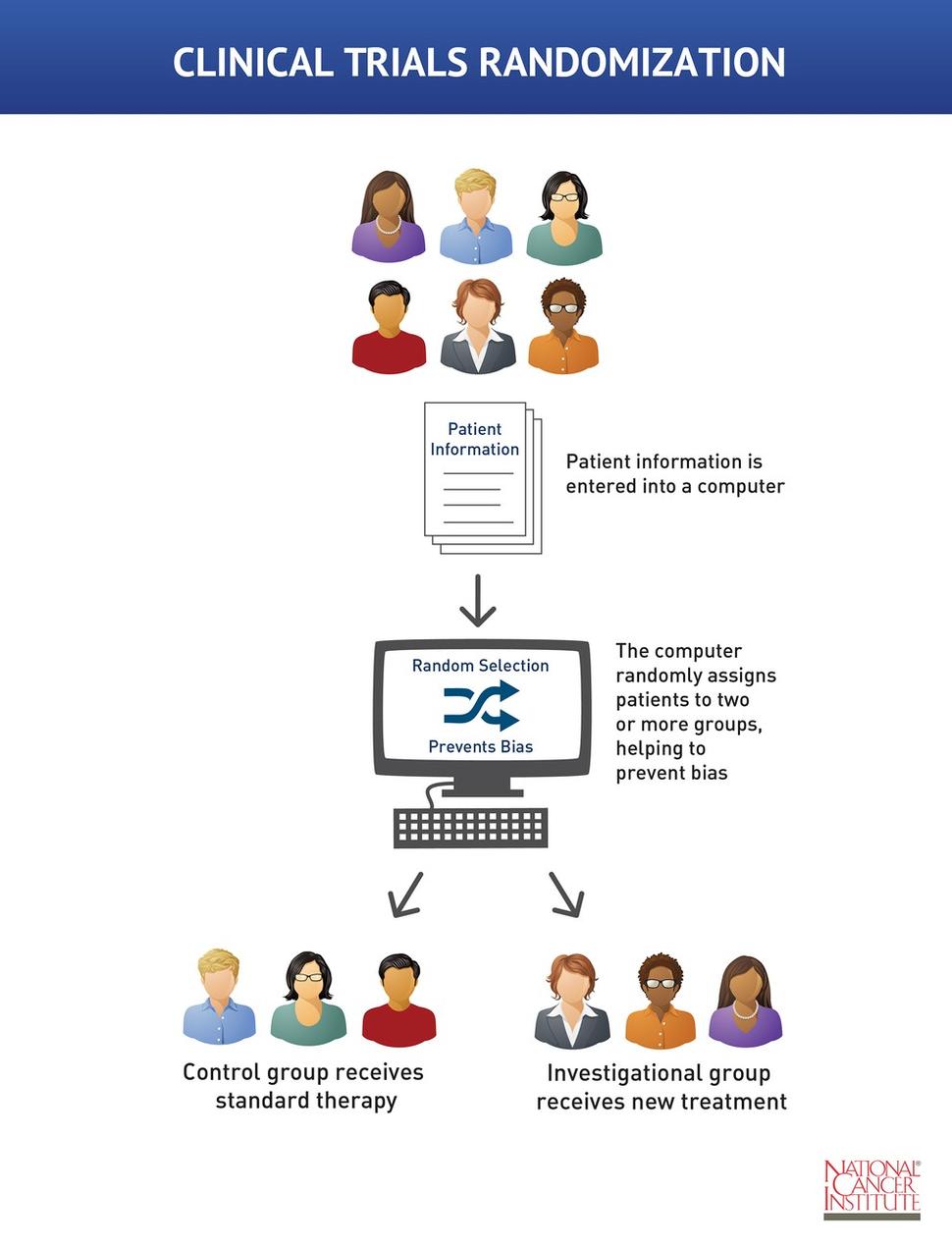 Randomization And Bias In Cancer Clinical Trials National Cancer Institute
Randomization And Bias In Cancer Clinical Trials National Cancer Institute
 Clinical Trials Cancer Survivors Cdc
Clinical Trials Cancer Survivors Cdc
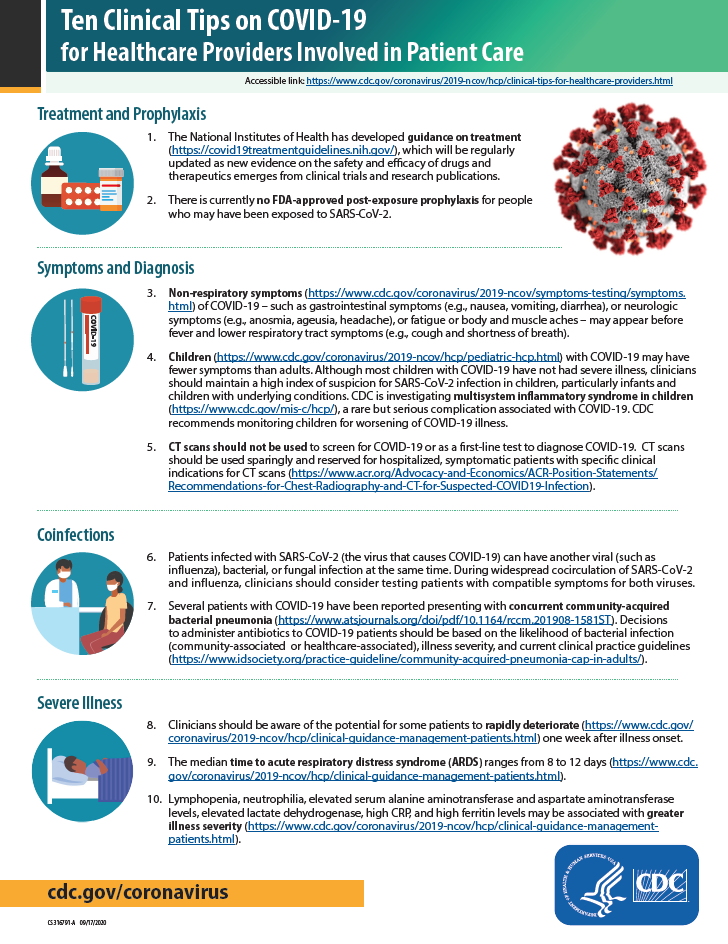 Ten Clinical Tips On Covid 19 For Healthcare Providers Involved In Patient Care Cdc
Ten Clinical Tips On Covid 19 For Healthcare Providers Involved In Patient Care Cdc
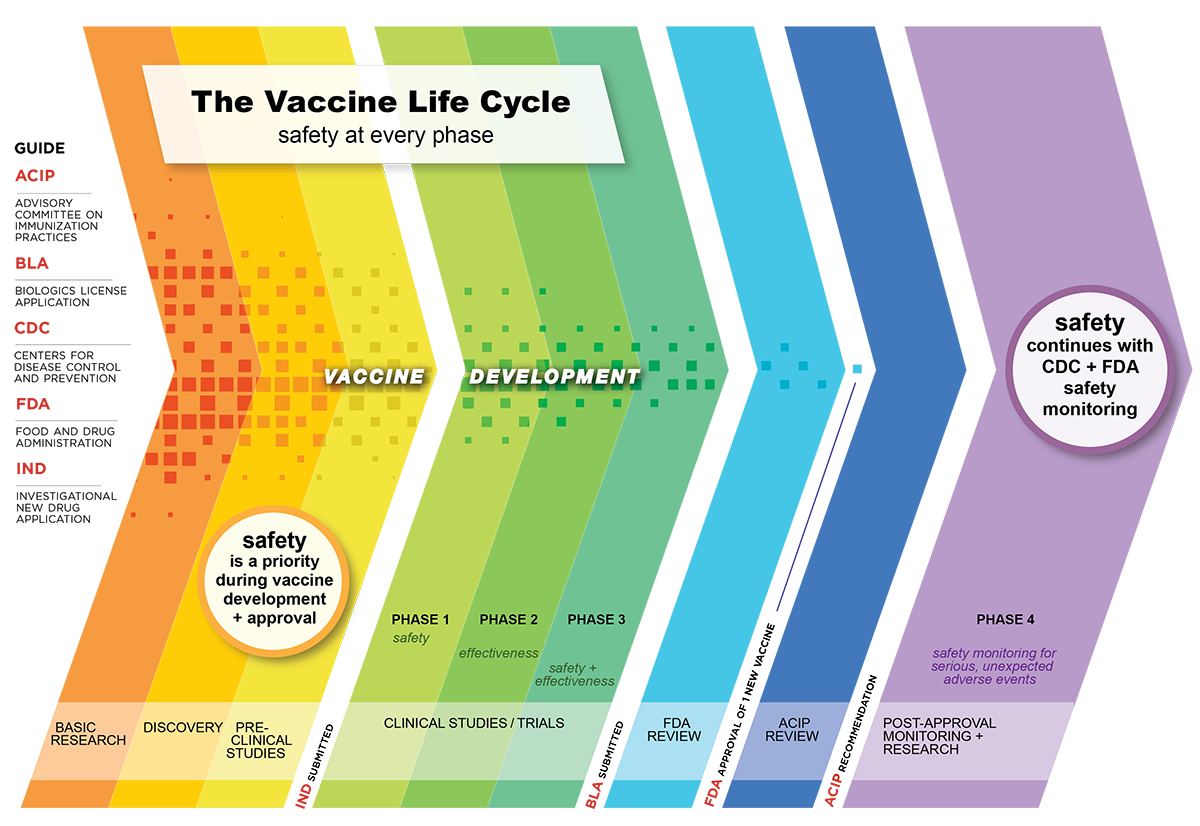 U S Vaccine Safety Overview History And How It Works Cdc
U S Vaccine Safety Overview History And How It Works Cdc
 Vaccine Safety What You Should Know National Foundation For Infectious Diseases
Vaccine Safety What You Should Know National Foundation For Infectious Diseases
 Africa Cdc Consortium For Covid 19 Vaccine Clinical Trials Concvact Africa Cdc
Africa Cdc Consortium For Covid 19 Vaccine Clinical Trials Concvact Africa Cdc
 Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
 Covid 19 Vaccine Training Modules
Covid 19 Vaccine Training Modules
Https Www Jhsph Edu Research Centers And Institutes Johns Hopkins Education And Research Center For Occupational Safety And Health M20 Handouts Howard Marcoem 10 October 2020 Final 4 Pdf
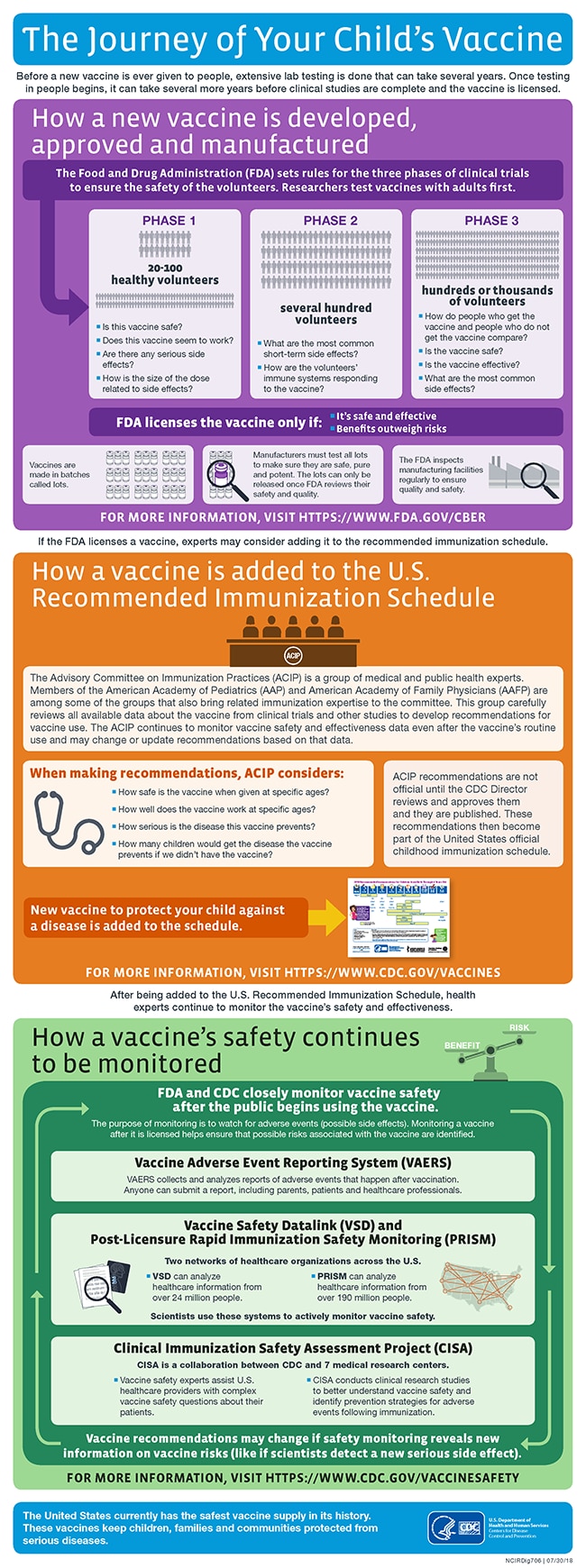
 The Journey Of Your Child S Vaccine Infographic Cdc
The Journey Of Your Child S Vaccine Infographic Cdc
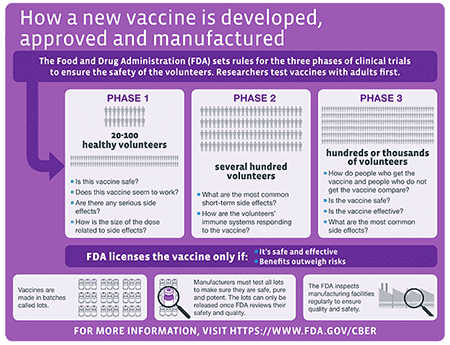 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
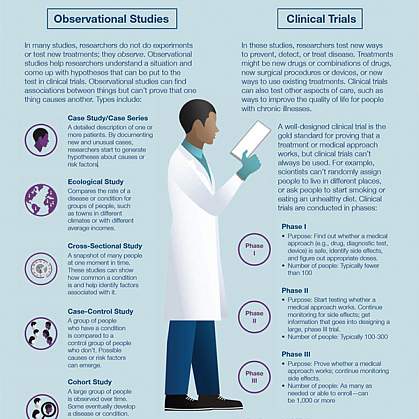 The Basics National Institutes Of Health Nih
The Basics National Institutes Of Health Nih
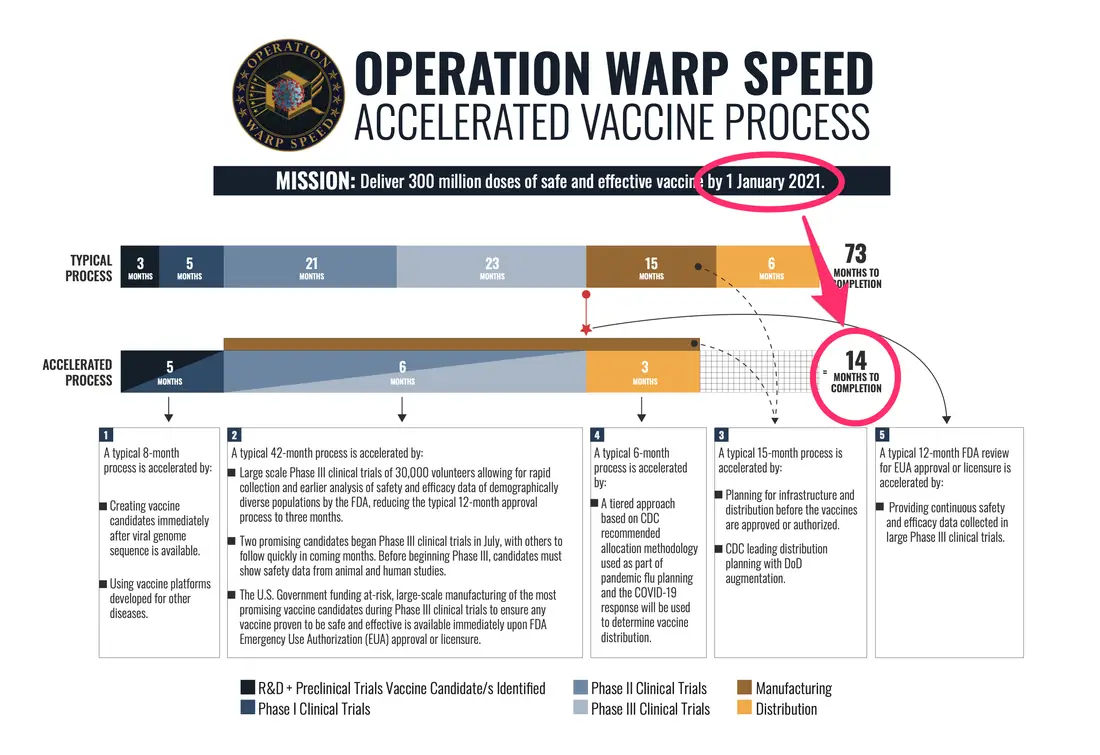
 Behind The Covid 19 Vaccine Approval Process Covid 19 Featured Health Topics Hackensack Meridian Health
Behind The Covid 19 Vaccine Approval Process Covid 19 Featured Health Topics Hackensack Meridian Health
Comments
Post a Comment